2023
Predictive IDH Genotyping Based on the Evaluation of Spatial Metabolic Heterogeneity by Compartmental Uptake Characteristics in Preoperative Glioma Using 18F-FET PET
Molecular markers are of increasing importance for classifying, treating, and determining the prognosis for central nervous system tumors. Isocitrate dehydrogenase (IDH) is a critical regulator of glucose and amino acid metabolism. Our objective was to investigate metabolic reprogramming of glioma using compartmental uptake (CU) characteristics in O -(2-18F-fluoroethyl)-l-tyrosine (FET) PET and to evaluate its diagnostic potential for IDH genotyping. Methods: Between 2017 and 2022, patients with confirmed glioma were preoperatively investigated using static 18F-FET PET. Metabolic tumor volume (MTV), MTV for 60%–100% uptake (MTV60), and T2-weighted and contrast-enhancing lesion volumes were automatically segmented using U-Net neural architecture and isocontouring. Volume intersections were determined using the Dice coefficient. Uptake characteristics were determined for metabolically defined compartments (central [80%–100%] and peripheral [60%–75%] areas of 18F-FET uptake). CU ratio was defined as the fraction between the peripheral and central compartments. Mean target-to-background ratio was calculated. Comparisons were performed using parametric and nonparametric tests. Receiver-operating-characteristic curves, regression, and correlation were used for statistical analysis. Results: In total, 52 participants (male, 27, female, 25; mean age ± SD, 51 ± 16 y) were evaluated. MTV60 was greater and distinct from contrast-enhancing lesion volume ( P = 0.046). IDH-mutated tumors presented a greater volumetric CU ratio and SUV CU ratio than IDH wild-type tumors ( P < 0.05). Volumetric CU ratio determined IDH genotype with excellent diagnostic performance (area under the curve [AUC], 0.88; P < 0.001) at more than 5.49 (sensitivity, 86%, specificity, 90%), because IDH-mutated tumors presented a greater peripheral metabolic compartment than IDH wild-type tumors ( P = 0.045). MTV60 and MTV were not suitable for IDH classification ( P > 0.05). SUV CU ratio (AUC, 0.72; P = 0.005) and target-to-background ratio (AUC, 0.68; P = 0.016) achieved modest diagnostic performance—inferior to the volumetric CU ratio. Furthermore, the classification of loss of heterozygosity of chromosomes 1p and 19q (AUC, 0.75; P = 0.019), MGMT promoter methylation (AUC, 0.70; P = 0.011), and ATRX loss (AUC, 0.73; P = 0.004) by amino acid PET was evaluated. Conclusion: We proposed parametric 18F-FET PET as a noninvasive metabolic biomarker for the evaluation of CU characteristics, which differentiated IDH genotype with excellent diagnostic performance, establishing a critical association between spatial metabolic heterogeneity, mitochondrial tricarboxylic acid cycle, and genomic features with critical implications for clinical management and the diagnostic workup of patients with central nervous system cancer.

Detection of recurrent high-grade glioma using microstructure characteristics of distinct metabolic compartments in a multimodal and integrative 18F-FET PET/fast-DKI approach - European Radiology
Objectives Differentiation between high-grade glioma (HGG) and post-treatment-related effects (PTRE) is challenging, but advanced imaging techniques were shown to provide benefit. We aim to investigate microstructure characteristics of metabolic compartments identified from amino acid PET and to eva…

2022
Fibrin-targeting molecular MRI in inflammatory CNS disorders - European Journal of Nuclear Medicine and Molecular Imaging
Background Fibrin deposition is a fundamental pathophysiological event in the inflammatory component of various CNS disorders, such as multiple sclerosis (MS) and Alzheimer’s disease. Beyond its traditional role in coagulation, fibrin elicits immunoinflammatory changes with oxidative stress response…

2021
atlasBREX: Automated template-derived brain extraction in animal MRI - Scientific Reports
Scientific Reports - atlasBREX: Automated template-derived brain extraction in animal MRI
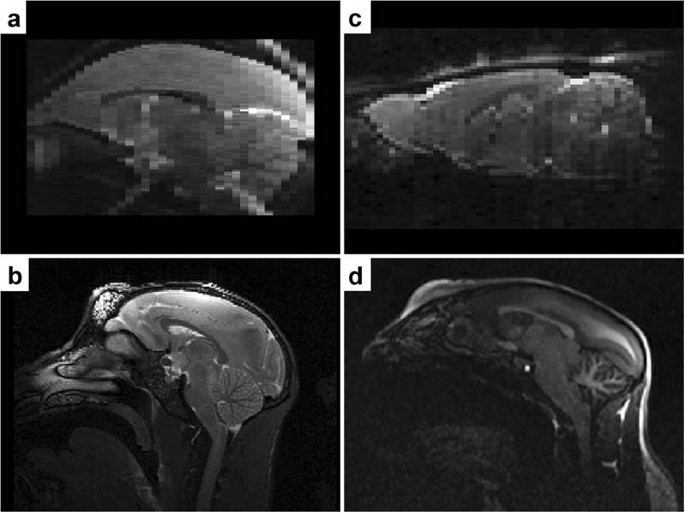
Quantitative biparametric analysis of hybrid 18F-FET PET/MR-neuroimaging for differentiation between treatment response and recurrent glioma - Scientific Reports
Scientific Reports - Quantitative biparametric analysis of hybrid 18F-FET PET/MR-neuroimaging for differentiation between treatment response and recurrent glioma
Google Scholar, ORCID-ID, Researchgate, Forschungsdatenbank-Charité
Current reviewing activity: European Radiology. NeuroImage.




